Critiques of NHST
Annoucements
- HW #2 due Friday
- No lab Friday (Veteran’s Day)
- Thursday (Nov 10) lecture will be pre-recorded
- Tonight at 5:30 pm
Recap
Does switching environments improve memory? On a standard memory task, participants remember 17.7 words (out of 30) \((\sigma = 3.2)\) after studying word lists for 9 minutes. You recruit a sample of 8 participants and make them study word lists in the student center. Every 3 minutes, they are required to stand up and move to a different room in the student center. After studying, participants are tested. On average, they remember 19.1 words \((s = 2.9)\).
Code
mu = 17.7; sigma = 3.2; n = 9; xbar = 19.1
sem = sigma/sqrt(n)
cv_pos = mu + 1.96*sem
cv_neg = mu - 1.96*sem
zstat = (xbar-mu)/sem
lower_bound = mu-3*sem
upper_bound = mu+3*sem
data.frame(M = c(lower_bound:upper_bound)) %>%
ggplot(aes(x = M)) +
stat_function(fun = function(x) dnorm(x, mean = mu, sd = sem),
geom = "line") +
stat_function(fun = function(x) dnorm(x, mean = mu, sd = sem),
geom = "area", fill = "purple", xlim = c(cv_pos, upper_bound)) +
stat_function(fun = function(x) dnorm(x, mean = mu, sd = sem),
geom = "area", fill = "purple", xlim = c(lower_bound, cv_neg)) +
geom_vline(aes(xintercept = xbar), color = "red", linetype = "dashed") +
scale_x_continuous(breaks = round(c(lower_bound:upper_bound))) +
labs(x = "Mean", y = "density", title = "Sampling distribution in original scale")+
theme_pubr()
Code
data.frame(M = c(-3:3)) %>%
ggplot(aes(x = M)) +
stat_function(fun = function(x) dnorm(x),
geom = "line") +
stat_function(fun = function(x) dnorm(x),
geom = "area", fill = "purple", xlim = c(1.96, 3)) +
stat_function(fun = function(x) dnorm(x),
geom = "area", fill = "purple", xlim = c(-3, -1.96)) +
geom_vline(aes(xintercept = zstat), color = "red", linetype = "dashed") +
scale_x_continuous(breaks = c(-3:3)) +
labs(x = "Z-statistic", y = "density", title = "Sampling distribution in Z-statistic units")+
theme_pubr()
The probability of getting this sample mean in this sampling distribution is:
Recap
You can test the hypothesis by comparing the sample mean \((\bar{X})\) to the mean under the null hypothesis \((\mu_0)\).
Alternatively, you can compare the the mean under the null hypothesis \((\mu_0)\) to the the sample mean \((\bar{X})\).
Code
mu = 17.7; sigma = 3.2; n = 9; xbar = 19.1
sem = sigma/sqrt(n)
cv_pos = xbar + 1.96*sem
cv_neg = xbar - 1.96*sem
zstat = (xbar-mu)/sem
lower_bound = mu-3*sem
upper_bound = xbar+3*sem
data.frame(M = c(lower_bound:upper_bound)) %>%
ggplot(aes(x = M)) +
stat_function(fun = function(x) dnorm(x, mean = xbar, sd = sem),
geom = "line") +
stat_function(fun = function(x) dnorm(x, mean = xbar, sd = sem),
geom = "area", fill = "purple", xlim = c(cv_pos, upper_bound)) +
stat_function(fun = function(x) dnorm(x, mean = xbar, sd = sem),
geom = "area", fill = "purple", xlim = c(lower_bound, cv_neg)) +
geom_vline(aes(xintercept = mu), color = "red", linetype = "dashed") +
scale_x_continuous(breaks = round(c(lower_bound:upper_bound))) +
labs(x = "Mean", y = "density", title = "Sampling distribution around sample mean")+
theme_pubr()
Recap: Errors
What kind of error could we have just made?
How do I assess the probability of errors?
Type I error (false positive) is equal to \(\alpha\). You, the researcher, choose the Type I error rate you are comfortable with.
Type II error (false negative) depends on making a guess about the effect size. This determines the mean of an alternative distribution
Code
mu = 17.7; sigma = 3.2; n = 9; xbar = 19.1
sem = sigma/sqrt(n)
cv_pos = mu + 1.96*sem
cv_neg = mu - 1.96*sem
zstat = (xbar-mu)/sem
lower_bound = mu-3*sem
upper_bound = mu+3*sem
data.frame(M = c(lower_bound:(upper_bound+5))) %>%
ggplot(aes(x = M)) +
stat_function(fun = function(x) dnorm(x, mean = mu, sd = sem),
geom = "line") +
stat_function(aes(fill = "Type I error"), fun = function(x) dnorm(x, mean = mu, sd = sem),
geom = "area", xlim = c(cv_pos, upper_bound),
alpha = .5) +
stat_function(aes(fill = "Type I error"), fun = function(x) dnorm(x, mean = mu, sd = sem),
geom = "area", xlim = c(lower_bound, cv_neg),
alpha = .5) +
stat_function(fun = function(x) dnorm(x, mean = mu+3, sd = sem),
geom = "line") +
geom_vline(aes(xintercept = cv_pos, color = "Type I error")) +
scale_x_continuous(breaks = seq(14,26,2), limits = c(14,26)) +
guides(color = "none") +
labs(x = "Mean", y = "density", title = "Sampling distribution in original scale", fill = NULL)+
theme_pubr()
Code
data.frame(M = c(lower_bound:(upper_bound+5))) %>%
ggplot(aes(x = M)) +
stat_function(fun = function(x) dnorm(x, mean = mu, sd = sem),
geom = "line") +
stat_function(aes(fill = "Type II error"), fun = function(x) dnorm(x, mean = mu+3, sd = sem),
geom = "area", xlim = c(lower_bound, cv_pos),
alpha = .5) +
stat_function(aes(fill = "Power"), fun = function(x) dnorm(x, mean = mu+3, sd = sem),
geom = "area", xlim = c(cv_pos, upper_bound+5),
alpha = .5) +
stat_function(fun = function(x) dnorm(x, mean = mu+3, sd = sem),
geom = "line") +
geom_vline(aes(xintercept = cv_pos, color = "Type I error")) +
scale_x_continuous(breaks = seq(14,26,2), limits = c(14,26)) +
scale_fill_brewer(palette = "Dark2") +
guides(color = "none") +
labs(x = "Mean", y = "density", title = "Sampling distribution in original scale", fill = NULL)+
theme_pubr()
How can power be increased?
Code
data.frame(M = c(lower_bound:(upper_bound+5))) %>%
ggplot(aes(x = M)) +
stat_function(fun = function(x) dnorm(x, mean = mu, sd = sem),
geom = "line") +
stat_function(fun = function(x) dnorm(x, mean = mu, sd = sem),
geom = "line") +
stat_function(aes(fill = "Type I error"),
fun = function(x) dnorm(x, mean = mu, sd = sem),
geom = "area",
xlim = c(cv_pos, upper_bound),
alpha = .5) +
stat_function(aes(fill = "Type I error"),
fun = function(x) dnorm(x, mean = mu, sd = sem),
geom = "area",
xlim = c(lower_bound, cv_neg),
alpha = .5) +
stat_function(aes(fill = "Type II error"),
fun = function(x) dnorm(x, mean = mu+3, sd = sem),
geom = "area",
xlim = c(lower_bound, cv_pos),
alpha = .5) +
stat_function(aes(fill = "Power"),
fun = function(x) dnorm(x, mean = mu+3, sd = sem),
geom = "area",
xlim = c(cv_pos, upper_bound+5),
alpha = .5) +
stat_function(fun = function(x) dnorm(x, mean = mu+3, sd = sem),
geom = "line") +
geom_vline(aes(xintercept = cv_pos,
color = "Type I error")) +
#scale_x_continuous(breaks = c(14:26), limits = c(14:26)) +
scale_fill_brewer(palette = "Dark2") +
guides(color = "none") +
labs(x = "Mean",
y = "density",
title = "Sampling distribution in original scale",
fill = NULL)+
theme_pubr()
\[Z_1 = \frac{CV_0 - \mu_1}{\frac{\sigma}{\sqrt{N}}}\]
- increase \(\mu_1\)
- decrease \(CV_0\)
- increase \(N\)
- reduce \(\sigma\)
Often the most challenging part of a power analysis is to settle on an effect size.
Past research can often provide some guidance, especially if a meta-analysis is available.
- Note that these are often over-estimates.
Sometimes a field might have standards regarding what counts as a meaningful effect (e.g., minimal clinically important difference).
Lacking this information, we can settle for more abstract benchmarks or rules of thumb about what are “small,” “medium”, and “large” effects.
But as we discussed, these benchmarks need to be contextualized in real-world outcomes.
Complicating these choices, effect sizes come in a variety of metrics.
Prevalence of false positives
Consider where \(\alpha\), \(\beta\) and power fall in this grid.
| Reject \(H_0\) | Do not reject | |
|---|---|---|
| \(H_0\) True | Type I Error | Correct decision |
| \(H_0\) False | Correct decision | Type II Error |
Prevalence of false positives
Consider where \(\alpha\), \(\beta\) and power fall in this grid.
| Reject \(H_0\) | Do not reject | |
|---|---|---|
| \(H_0\) True | \(\alpha\) | \(1-\alpha\) |
| \(H_0\) False | \(1-\beta\) | \(\beta\) |
What is the sum of these cells?
Prevalence of false positives
Figuring out how common Type I and Type II errors are in a literature requires an additional piece of information:
- How often \(H_0\) is true.
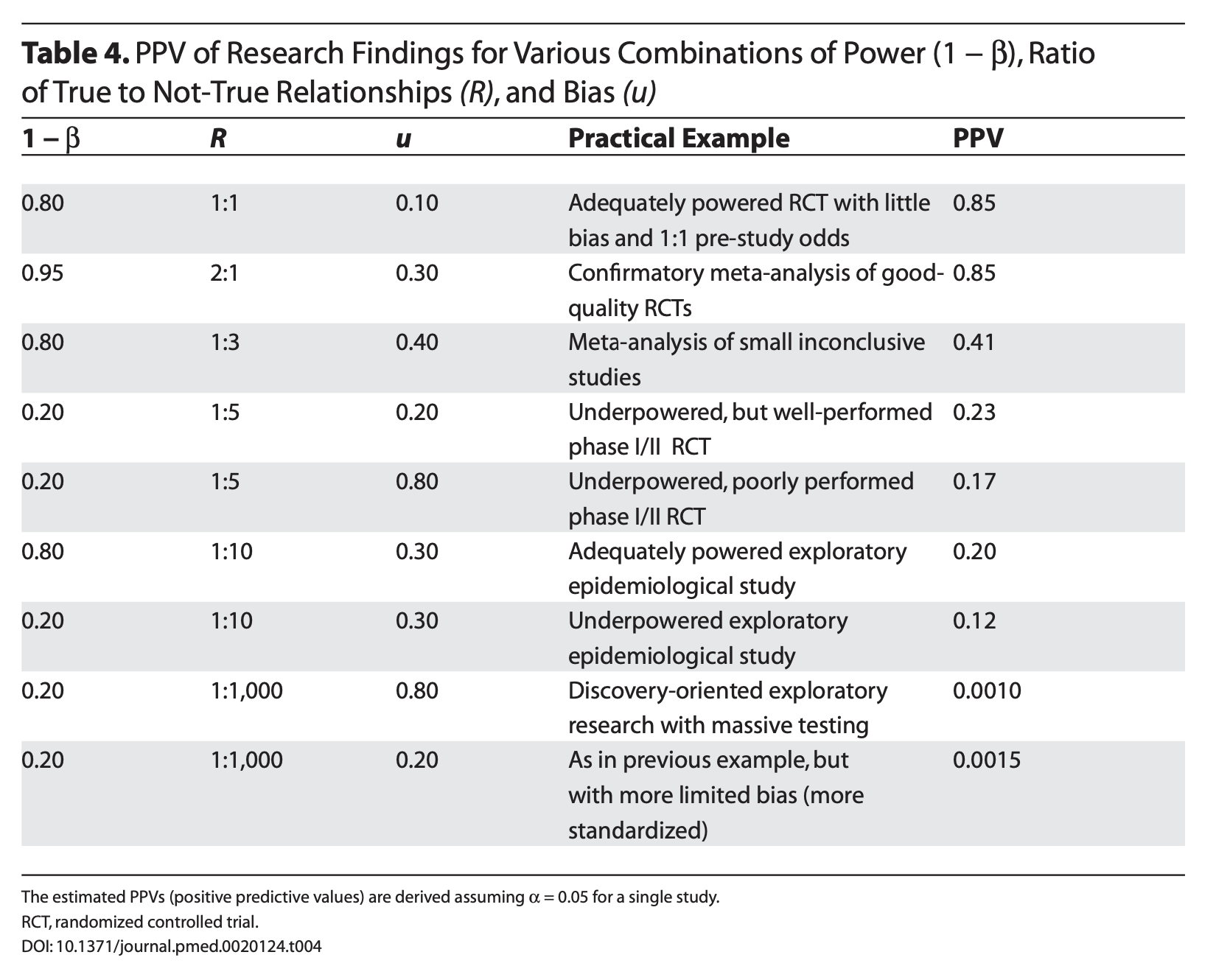
Ioannidis (2005) demonstrates that given R (the ratio of \(H_0\) false over \(H-0\) true), we can calculate the positive predictive value of a finding, which is the likelihood that \(H_1\) is true given we found a significant result:
\[\frac{(1-\beta)R}{(1-\beta)R+\alpha}\]
Let’s make some assumptions. If psychology studies use \(\alpha = .05\) and are good at achieving adequate power ( \(1-\beta = .80\) ), how does the choice of hypothesis affect PPV?
Code
ppv_fun = function(R, beta = .20, alpha = .05){
ppv = ((1-beta)*R)/((1-beta)*R + alpha)
return(ppv)
}
data.frame(prob = seq(0,1, by = .01)) %>%
mutate(ratio = prob/(1-prob),
ppv = ppv_fun(ratio)) %>%
ggplot(aes(x = prob, y = ppv)) +
geom_line() +
scale_x_continuous(expression("Probability that"~H[1]~"is true")) +
scale_y_continuous("Positive Predictive Value")+
theme_pubr(base_size = 20)
However, there are many reasons to think that psychology studies are underpowered. Typical power may be closer to .5 or even worse.
Code
data.frame(prob = seq(0,1, by = .01)) %>%
mutate(ratio = prob/(1-prob),
ppv = ppv_fun(ratio),
ppv_low = ppv_fun(ratio, beta = .5)) %>%
ggplot(aes(x = prob)) +
geom_line(aes(y = ppv, color = ".80")) +
geom_line(aes(y = ppv_low, color = ".50")) +
scale_x_continuous(expression("Probability that"~H[1]~"is true")) +
scale_y_continuous("Positive Predictive Value")+
scale_color_discrete("Power")+
theme_pubr(base_size = 20)
In addition to being under-powered, there’s recognition that research practices have inflated family-wise error, making \(\alpha\) much bigger than it should be.
Code
data.frame(prob = seq(0,1, by = .01)) %>%
mutate(ratio = prob/(1-prob),
ppv = ppv_fun(ratio),
ppv_low = ppv_fun(ratio, beta = .5),
ppv_alp = ppv_fun(ratio, alpha = .20),
ppv_low_alp = ppv_fun(ratio, beta = .5, alpha = .20)) %>%
ggplot(aes(x = prob)) +
geom_line(aes(y = ppv, color = ".80", linetype = ".05")) +
geom_line(aes(y = ppv_low, color = ".50", linetype = ".05")) +
geom_line(aes(y = ppv_alp, color = ".80", linetype = ".20")) +
geom_line(aes(y = ppv_low_alp, color = ".50", linetype = ".20")) +
scale_x_continuous(expression("Probability that"~H[1]~"is true")) +
scale_y_continuous("Positive Predictive Value")+
scale_color_discrete("Power")+
scale_linetype_discrete("Alpha") +
theme_pubr(base_size = 20)
Critiques of NHST
Many will describe the current “replication crisis” or “reproducibility crisis” or “open science movement” as tracing its beginnings to 2011, mostly due to:
Critiques of NHST
But the reality is that NHST has always had its critics. And it’s not for lack of eloquence that they have been ignored…
“What’s wrong with [NHST]? Well, among many other things, it does not tell us what we want to know, and we so much want to know what we want to know that, out of desperation, we nevertheless believe that it does!” – Cohen (1994)
What kind of mess have we gotten ourselves into?
\(p < .05\) as a condition for publication
Publication as a condition for tenure
Novelty as a condition for publication in top-tier journals
Institutionalization of NHST
High public interest in psychological research
Unavoidable role of human motives: fame, recognition, ego
What kind of science have we produced?
\(p < .05\) as a primary goal
Publication bias: “Successes” are published, “failures” end up in file drawers
Overestimation of effect size in published work
Underestimation of complexity (why did the failures occur?)
Underestimation of power
Inability to replicate
Settling for vague alternative hypotheses: “We expect a difference”
What kind of science have we produced?
Dichotomous thinking (based on \(p\) ): research either “succeeds” or “fails” to find the expected difference
No motivation to pursue failures to reject the null
Harvesting (mostly) the low-hanging fruit in science to publish quickly and often.
Weak theory
- Low precision (“differences” are enough)
- No non-nil null hypotheses
- Weak, slow progress as a science
Focusing on \(p\) -values
Let’s start with some intuition.
Imagine you conduct two studies. Assuming your alpha is .05 and the null is true, what is the probability that the first study or the second study or both studies yield significant results?
- Less than 5%?
- Exactly 5%?
- More than 5%?
Focusing on \(p\) -values
Imagine rolling a die.
What’s the probability you roll a 2?
- \(P(6) = 1/20 = 16.7\%\)
If you roll the die twice, what’s the probability that you get a 2 at least once? \(30.6\%\)
If you roll the die 5 times, what’s the probability that you get a 2 at least once? \(59.8\%\)
Roll the die enough times, and you’ll get a 2 eventually. Significance testing when the null is true is like rolling a 20-sided die.
False Positive Psychology
Simmons et al. (2011) pointed out that each study is not a single roll of the die.
Instead, each study, even those with a single statistical test, might represent many rolls of the die.
- Researcher degrees of freedom: Decisions that a researcher makes that change the statistical test.
- Examples:
- Additional dependent variables
- Tests with and without covariates
- Data peeking
- Examples:
Each time I see how a decision affected my result, I am rolling the dice again.
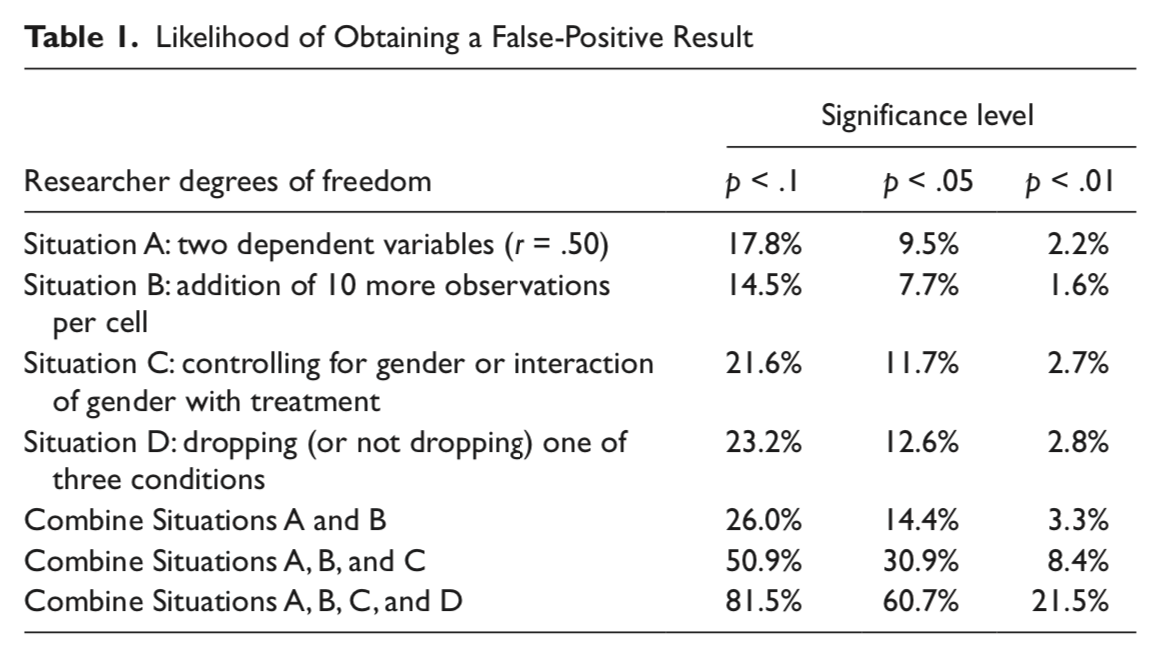
p-hacking
p-hacking: collecting or selecting data or statistical analyses until non-significant results become significant.
Prior to 2011, this was common practice. In fact, it was often taught as best practices.
- “Explore your data.”
- “Understand your data.”
- “Test sensitivity…”
We should recognize now that this inflates Type I error.
p-curve (2013)
One of the most useful discoveries of the early reform movement was the expected behavior of p-values under different conditions. We refer broadly to these analyses as the p-curve, because they describe the distribution of expected p-values in different scenarios.
click here for lots of extra information!
Let’s start with the main two:
- The null hypothesis is true.
- The null hypothesis is false.
Let’s simulate what would happen if we repeated an experiment 10,000 times in a world in which the null hypothesis was true…
\(H_O\) true \((N = 30, \mu = 0, \sigma = 1)\)
Code
set.seed(201112)
# number of simulations
sims = 10000
# sample size
N = 30
# create empty vector for storing p-values
p_values = numeric(sims)
# loop
for(i in 1:sims){
# draw sample
sample = rnorm(n = N,
mean = 0,
sd = 1)
sample_mean = mean(sample)
sample_sd = sd(sample)
se_mean = sample_sd/sqrt(N)
z_stat = (sample_mean-0)/se_mean
sample_p = pnorm(q = abs(z_stat),
lower.tail = F)
p_values[i] = sample_p
}
\(H_O\) false \((N = 30, \mu = 0.25, \sigma = 1)\)
Code
set.seed(201112)
# create empty vector for storing p-values
p_values = numeric(sims)
# loop
for(i in 1:sims){
# draw sample
sample = rnorm(n = N,
mean = .25,
sd = 1)
sample_mean = mean(sample)
sample_sd = sd(sample)
se_mean = sample_sd/sqrt(N)
z_stat = (sample_mean-0)/se_mean
sample_p = pnorm(q = abs(z_stat),
lower.tail = F)
p_values[i] = sample_p
}
| ### \(H_O\) false \((N = 30, \mu = 0.50, \sigma = 1)\) |
| ::: {.cell hash=‘13-critiques_cache/revealjs/unnamed-chunk-15_9b05f3b893d74c14725a4d8d38aaf5f5’} |
{.r .cell-code code-fold="true"} set.seed(201112) # create empty vector for storing p-values p_values = numeric(sims) # loop for(i in 1:sims){ # draw sample sample = rnorm(n = N, mean = .50, sd = 1) sample_mean = mean(sample) sample_sd = sd(sample) se_mean = sample_sd/sqrt(N) z_stat = (sample_mean-0)/se_mean sample_p = pnorm(q = abs(z_stat), lower.tail = F) p_values[i] = sample_p } ::: |
::: {.cell} ::: {.cell-output-display}  ::: ::: ::: ::: |
\(H_O\) false \((N = 30, \mu = 0.50, \sigma = 1)\)
These distributions describe what we expect p-values to look like in the long run when a given hypothesis is true or false.
But note: what’s an important assumption in these simulations?
- What if experimental studies were not independent of one another?
Imagine a research has a good idea. They run an experiment. One of two things happen:
- They get a significant result.
- They do not get a significant result.
Do they repeat the study? Say the motivated researcher tries again, only if they don’t find a significant result, but doesn’t try again if the results come out the way they expect. What happens?
\(H_O\) true \((N = 30, \mu = 0, \sigma = 1)\) and non-independence
Code
set.seed(201112)
# create empty vector for storing p-values
p_values = numeric(sims)
# loop
for(i in 1:sims){
try = 1
sample_p = 1
# while these statements are true,
# keep going
while(sample_p >= .05 & try <= 2){
sample = rnorm(n = N,
mean = 0,
sd = 1)
sample_mean = mean(sample)
sample_sd = sd(sample)
se_mean = sample_sd/sqrt(N)
z_stat = (sample_mean-0)/se_mean
sample_p = pnorm(q = abs(z_stat),
lower.tail = F)
try = try+1
}
p_values[i] = sample_p
}
\(H_O\) true \((N = 30, \mu = 0, \sigma = 1)\) and non-independence
Code
set.seed(201112)
# create empty vector for storing p-values
p_values = numeric(sims)
# loop
for(i in 1:sims){
try = 1
sample_p = 1
# while these statements are true,
# keep going
while(sample_p >= .05 & try <= 3){
try = try+1
sample = rnorm(n = N,
mean = 0,
sd = 1)
sample_mean = mean(sample)
sample_sd = sd(sample)
se_mean = sample_sd/sqrt(N)
z_stat = (sample_mean-0)/se_mean
sample_p = pnorm(q = abs(z_stat),
lower.tail = F)
}
p_values[i] = sample_p
}
Code
phack_data = data.frame(sim = 1:sims, p=p_values,
type = "p-hack")
set.seed(201112)
# create empty vector for storing p-values
p_values = numeric(sims)
# loop
for(i in 1:sims){
# draw sample
sample = rnorm(n = N,
mean = .25,
sd = 1)
sample_mean = mean(sample)
sample_sd = sd(sample)
se_mean = sample_sd/sqrt(N)
z_stat = (sample_mean-0)/se_mean
sample_p = pnorm(q = abs(z_stat),
lower.tail = F)
p_values[i] = sample_p
}
nonp_data = data.frame(sim = 1:sims, p=p_values,
type = "true H1")
full_join(phack_data, nonp_data) %>%
ggplot(aes(x = p, fill = type)) +
geom_density(alpha = .4) +
scale_x_continuous(limits = c(0, .1)) +
theme_bw(base_size = 16) +
labs(x = "p-value", y = "frequency",
title = "Two extra tries")
p-curve
p-curve made it clear that if our null hypotheses were indeed false, we would most often see very small p-values.
Importantly, this also signalled that literatures (or papers) that contained lots of p-values between .01 and .05 were relatively unlikely, unless there were some fishing expeditions going on.
The publication of “False Positive Psychology” and the p-curve paper, following the claim by Ioannidis (2005) that as many as half of published findings are false prompted researchers to take a second look at the “knowns” in our literatures.
If we can demonstrate these “known” effects, then we’re ok. Our effects are most likely true.
And if that had happened, we probably wouldn’t have two lectures in this class dedicated to problems with NHST and how to address them.
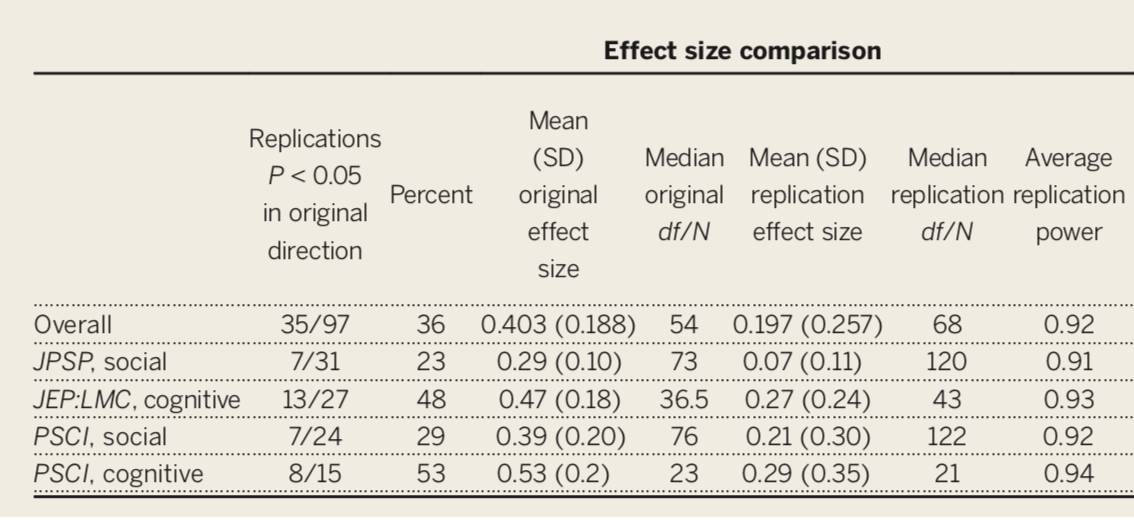
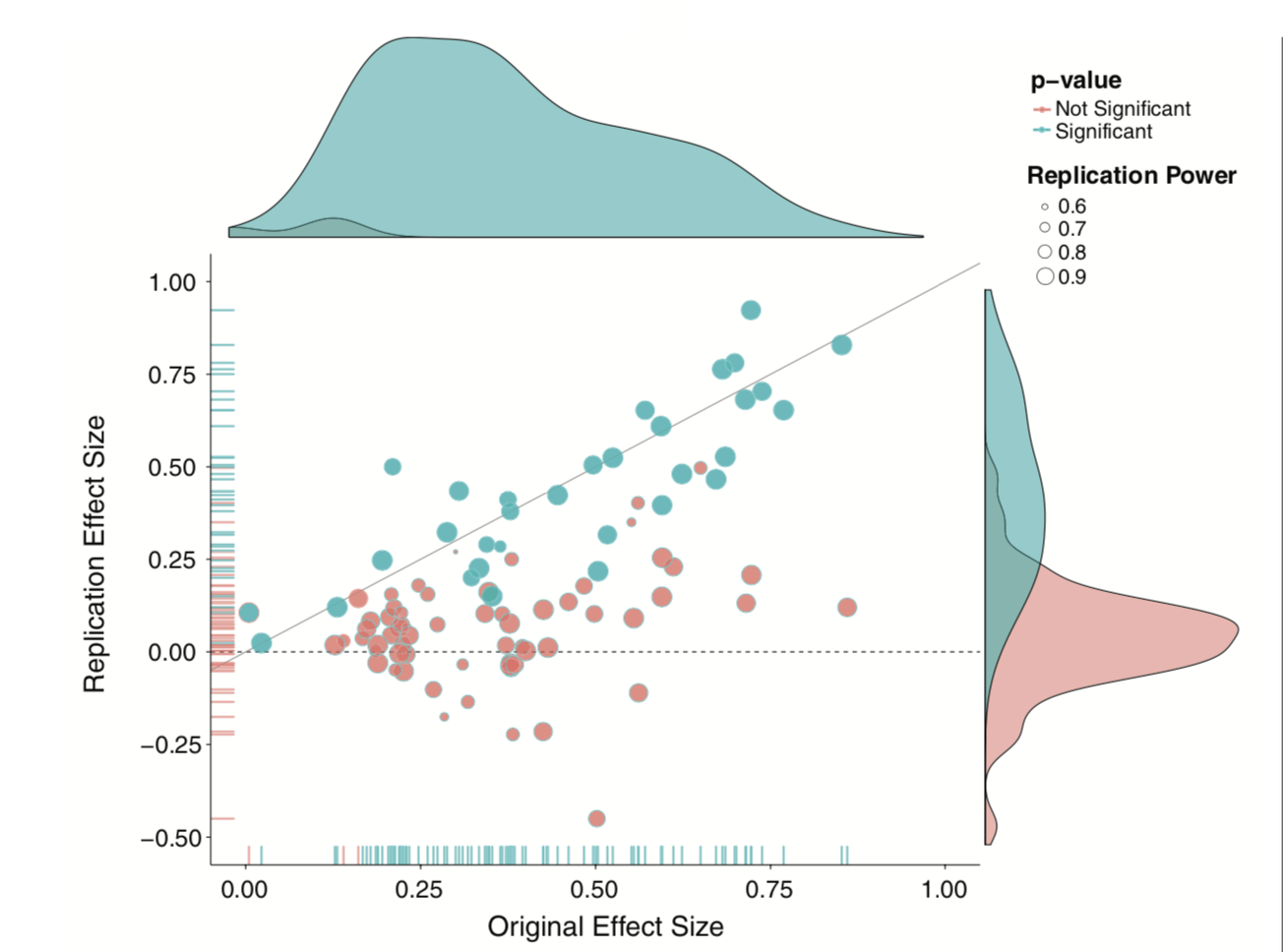
The inability to replicate published research has been viewed as especially troubling.
- This has been a long-standing concern, but the poster child is undoubtedly “Estimating the reproducibility of psychological science” by the Open Science Collaboration (Science, 2015, 349, 943).
Only 36% of the studies were replicated, despite high power and claimed fidelity of the methods.
Why is it so hard to replicate?
- Poor understanding of context necessary to produce most effects
- We do not recognize the boundary conditions of effects especially when the limiting conditions are kept constant
- Incomplete communication of the necessary conditions
- Akin to reading just the first few ingredients for a recipe and then trying to duplicate the dish.
Why is it so hard to replicate?
Sparse communication fosters belief by others that effects are simpler and easier to produce than they really are.
The reality is that key elements have been left out:
specific methodological or analytic details
and the tests run before and after the ones that were published.
Regarding replications, there’s an additional methodological challenge to grapple with.
Example: The original study \((N = 25)\) found a significant effect, \(\bar{X} = 20\), 95% CI \([5, 35]\). The second study \((N = 40)\) does not find a significant effect, \(\bar{X} = 10\), 95% CI \([-1, 21]\). Did the second study replicate the first?
There is disagreement about what counts as “replicating the effect.” - Both studies significant? - Significant difference in effect size?
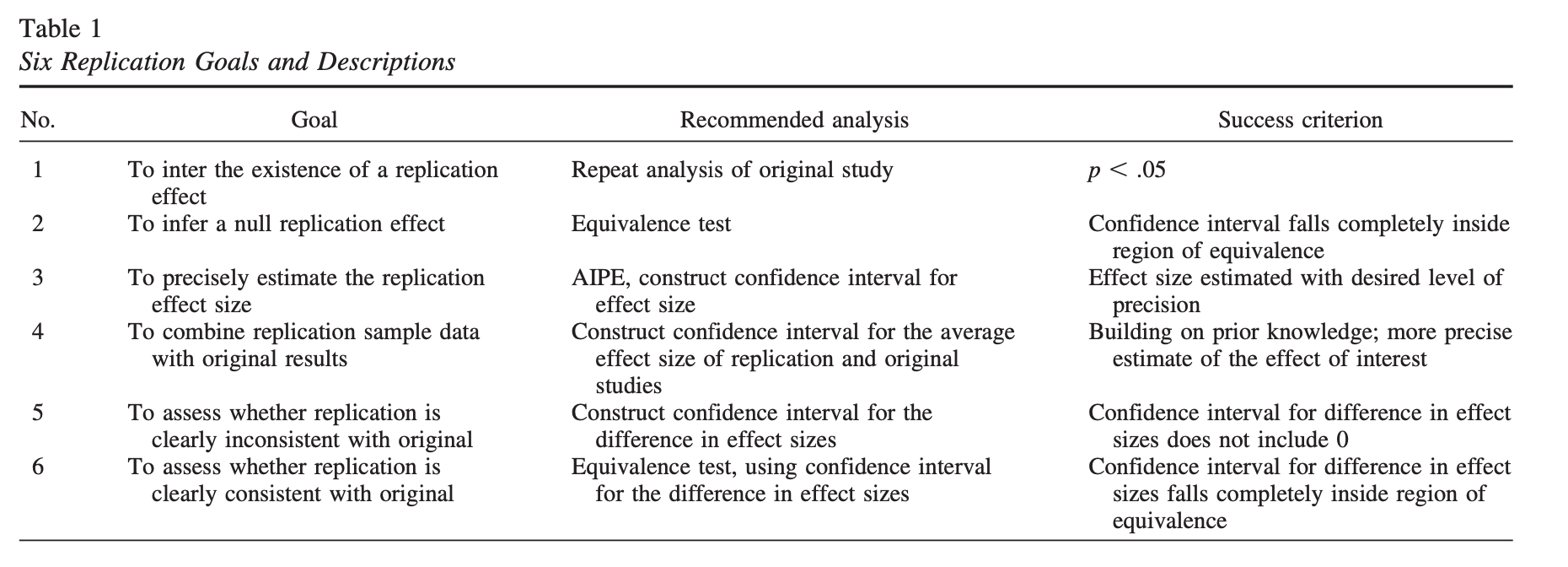
Anderson & Maxwell (2016)
Ultimately, much of the problems, both pre-2011 and since, have stemmed from an over-reliance on p-values (Anderson, 2019).
This is a point that bears repeating, early and often:
Science cannot use a single metric to evaluate the existence or importance of effects and theories.
- Detectives don’t make a case on a single clue.
- Doctors don’t diagnosis illness using a single symptom.
- You shouldn’t rely on a single number either.
- Plus, we need to stop relying on single studies.
What kind of challenges will need to be addressed?
Institutionalization of CI and ES but not \(p\)
Journals must change their values
How do we shift media values? Replication is not “sexy.”
How do we shift academic values? What will be the impact on tenure? How do we re-calibrate “productivity?” Citation indices?
The inability to replicate much work has been viewed as a singular failure of psychology as a science.
But is that the best way to view it?
As data, the replication crisis, it is potentially quite informative and suggests some importance features of psychology as a science.
At a minimum, it tells us we don’t understand a phenomenon as well as we think we do. That is hardly a failure of science, unless we don’t take the next steps to resolve our ignorance.
Failures to replicate are surprising, even troubling. We think of them as unfortunate occurrences. Bad luck even. But should they be viewed that way?
Surprises in science have a long history of playing a key role in scientific progress.
A failure to replicate should be viewed as particularly interesting.
It is time to insist that science does not progress by carefully designed steps called “experiments” each of which has a well-defined beginning and end. Science is a continuous and often disorderly and accidental process. A first principle not formally recognized by scientific methodologists: when you run onto something interesting, drop everything else and study it. –B. F. Skinner (1968) A case history in scientific method
Next time…
Open science and catching up
